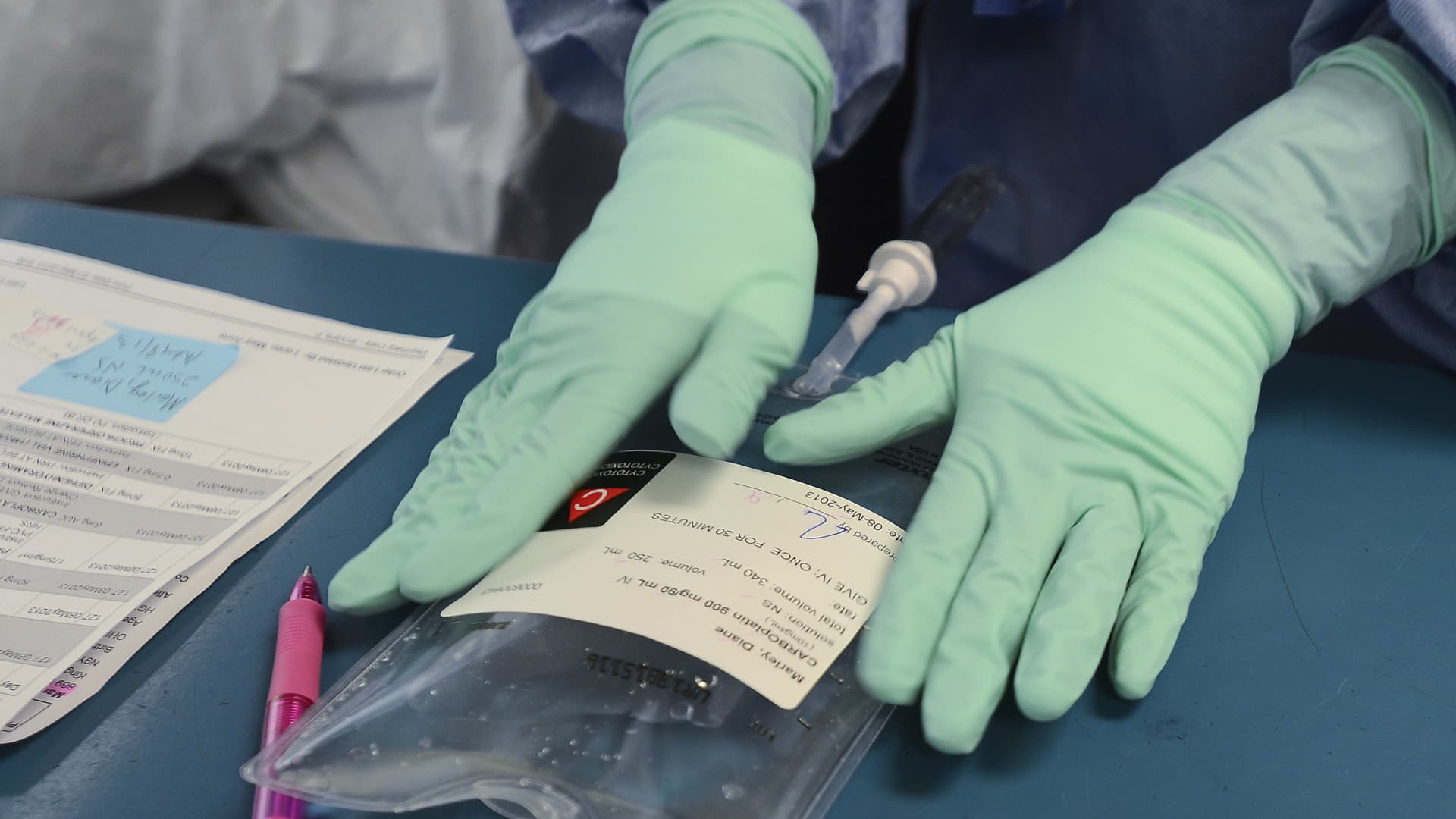
WINDSOR, ON – MAY 8: Registered Pharmacy Technician Dawn Deslippe carefully labels Diane’s dose of Carboplatin, one of two chemo drugs she will receive on this visit. Every step of the process involves verification from at least two people. The hospital now prepares the chemo drugs themselves rather than getting them pre-mixed.
Diane Marley, 48 is a cancer patient at Windsor Regional Hospital. She was diagnosed with breast cancer in December. She is finishing up her chemo regimen in the next few weeks. She is one of hundreds of Ontario cancer patients who received diluted chemotherapy in the last year and who are still undergoing treatment to beat the disease. (Richard Lautens/Toronto Star via Getty Images)
Richard Lautens | Toronto Star | Getty Images
The Food and Drug Administration – faced with a national shortage of more than a dozen cancer medications – is considering allowing the temporary importation of chemotherapy drugs from overseas manufacturers that are not currently approved to distribute in the United States, an agency spokesperson told CNBC.
The FDA did not say which manufacturers would be potential candidates for permitting temporary importation of those drugs until approved manufacturers are able to meet patients’ needs.
But, “in these cases, we very carefully assess the overseas product for quality, making sure that its safe for U.S. patients,” the spokesperson said.
The FDA in the past has taken similar action to loosen restrictions on imports when faced with drug shortages. In the summer of 2022, the FDA allowed the importation of baby formula from non-agency-approved manufacturers when there was a serious shortage of formula in the U.S.
The American Society of Clinical Oncology anticipates the shortages will continue through June but then subside particularly if the FDA lifts import restrictions, according to Dr. Julie Gralow, that group’s chief medical officer.
“We’re hoping and estimating that once we get through the next month that we will have a more stable supply,” Gralow said.
At least 14 cancer drugs are currently in short supply across the U.S.
But doctors at hospitals around the country say the situation is particularly acute for two drugs — cisplatin and carboplatin — because they are so fundamental and widely used in cancer treatment.
The World Health Organization has said cisplatin and carboplatin are essential for basic health care.
Intas Pharmaceuticals, one of the biggest makers of those drugs, temporarily shut down production and it is not clear when the company will resume manufacturing.
Up to 20% of cancer patients rely on platinum-based chemotherapy drugs such as cisplatin and carboplatin for treatment, according to the National Cancer Institute.
And more than 100,000 Americans were diagnosed in 2022 with cancers that may be treated with carboplatin or cisplatin, generic drugs that have been on the market for decades, the American Society of Clinical Oncology says.
Those drugs are used to treat a wide range of diseases including testicular, ovarian, breast, lung, bladder and head-and-neck cancers.
Shortages of the drugs have forced some hospitals to ration the medications by reducing doses to extend their supply, and to prioritize patients who would benefit the most from treatment.
Some cancer patients could die if the shortages are not quickly resolved, doctors said.
“The lawmakers in the country need to understand that this is a big problem at this point, where unless something changes in the next few weeks, this can lead to a big national emergency from a patient and health care standpoint,” said Dr. Abdul Rafeh Naqash, a doctor at the Stephenson Cancer Center at the University of Oklahoma.
Naqash said his facility is on the verge of running out of carboplatin. He said the shortages are a national security issue that needs to be quickly addressed.
“Things have been getting worse on the ground. Something has to happen and change immediately,” said Naqash, who specializes in lung cancer.
He said he recently had to inform a patient that they will not receive carboplatin due to the shortage.
Such conversations will likely become more common in the coming weeks if relief does not come, Naqash said.
Naqash said he does not understand why the U.S. does not have a national stockpile of these medications to fill the gap in emergency situations.
Philip Schwieterman, director of oncology and infusion services at the University of Kentucky health system, said, “If I go in the grocery store and I want a kiwi, there are usually kiwis there.”
“It boggles my mind that if I want some cisplatin, I can’t get cisplatin even though it saves lives,” Schwieterman said.
‘A cascading drug shortage’
The cisplatin and carboplatin shortages stem from the temporary shutdown of manufacturing for the U.S. market at a plant in India run by Intas Pharmaceuticals.
Intas decided to halt manufacturing after an FDA inspection found a “cascade of failure” in the facility’s quality control unit late last year.
Intas, which is headquartered in Ahmedabad, India, distributes cisplatin and carboplatin in the U.S. through its subsidiary, Accord Healthcare.
When the cisplatin shortages began in February, many patients switched to carboplatin, which is considered a sister drug, said Marc Phillips, who manages the inpatient pharmacy supply chain for WVU Medicine, the largest health-care system in West Virginia.
That shift has “led into what we consider a cascading drug shortage,” Phillips said.
“One shortage has now caused another,” he said.
Fresenius Kabi, Hikma Pharmaceuticals, Teva and Pfizer produce the medications, but those companies have been unable to keep up with demand since the Intas plant went offline.
Intas is working on a plan with the FDA to restart manufacturing.
But no date has been confirmed, said company spokesperson Emily King.
When the plant does restart, production will prioritize drugs based on medical necessity, King said.
She noted that the FDA’s drug shortage staff and compliance office have identified carboplatin and cisplatin as a medical necessity for the U.S. market.
The FDA spokesperson said Intas has begun releasing into the U.S. doses of cisplatin and carboplatin that were previously on hold due to a testing and verification process.
Ensuring cancer treatments continue production
Dr. Karen Knudsen, CEO of the American Cancer Society, said the shortages highlight a long-standing economic problem in the generic drug market.
Manufacturers are hesitant to invest more money in producing low-cost drugs like cisplatin and carboplatin, which makes them vulnerable to shortages when a plant goes down, Knudsen said.
Knudsen fears the U.S. is entering a cycle of cancer drug shortages if the federal government and industry do not act together to fix the problem.
“We need it to be financially viable for manufacturing to be able to produce effective, affordable cancer therapies,” she said.
Knudsen said demand for these drugs will increase in the coming years as the population ages because older individuals are at higher risk for cancer.
And medications such as carboplatin and cisplatin use precious metals – platinum – that are heavily sourced from South Africa and Russia.
The World Platinum Investment Council is forecasting a major deficit of the precious metal this year due in part to disruptions in South Africa caused by an electricity shortage and operational problems in Russia due to sanctions over the Kremlin’s invasion of Ukraine.
Drugmakers are required to inform the FDA about manufacturing disruptions six months in advance or as soon as they are able. Knudsen said the early warning system doesn’t seem to be working effectively.
“The fact that we’re sitting here right now talking about this cancer shortage tells us that the early warning system was either not activated early enough, or there are not enough manufacturers to be able to to overcome the supply chain issue,” she said.
The FDA is working with the company to increase supply to meet patient demand, the agency spokesperson said.
A trio of Michigan Democratic lawmakers, Sens. Debbie Stabenow and Gary Peters, Rep. Elissa Slotkin, in a letter last month urged FDA Commissioner Dr. Robert Califf to “utilize all of its existing authorities to mitigate this dire shortage.”
The letter said that Congress is working on long-term solutions to drug shortages, which have been a problem for years.
