For about four hours of arguments, a federal judge in Texas asked questions that suggested he is seriously considering undoing the US Food and Drug Administration’s approval of a medication abortion drug and the agency’s moves to relax the rules around its use.
But the judge, US District Judge Matthew Kacsmaryk, an appointee of former President Donald Trump, also indicated he was thinking through scenarios in which he could keep the drug’s 2000 approval intact while blocking other FDA rules.
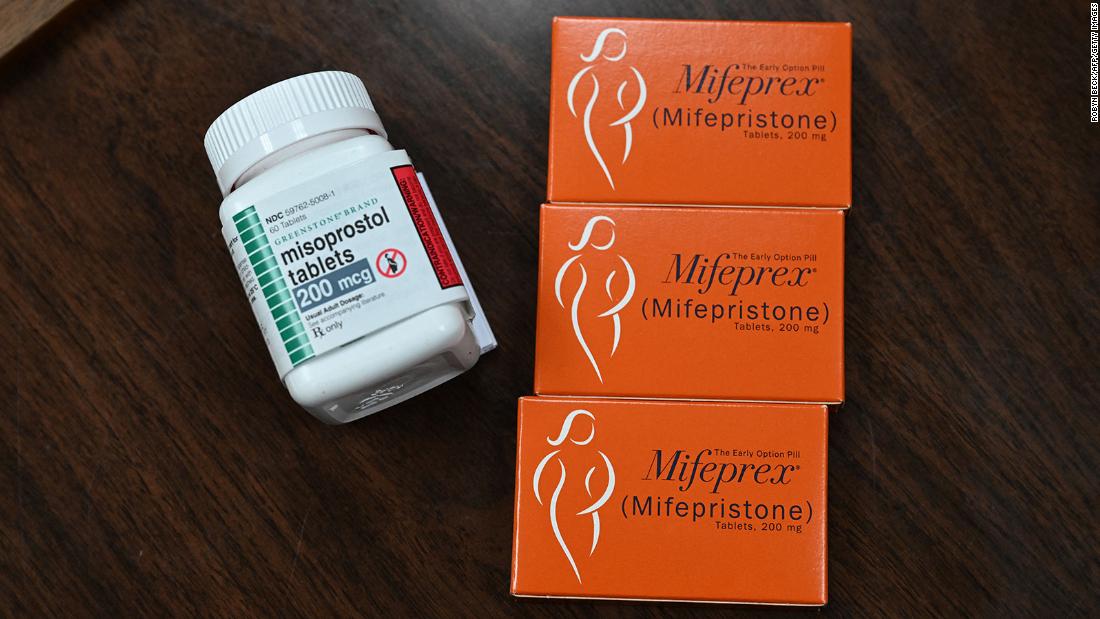
Anti-abortion doctors and medical associations are seeking a preliminary injunction that would require the FDA to withdraw or suspend its approval of the drug, mifepristone, and that would block the agency’s more recent regulatory changes making the pills more accessible.
Here are some of the big takeaways:
Judge focused on FDA’s process for approving abortion pills: Kacsmaryk showed a particular interest in the arguments by the abortion opponents that the FDA approved mifepristone in an unlawful way.
He zeroed in on a claim by the abortion foes that the studies that the FDA looked at when deciding whether to approve the drug did not match the conditions under which the agency allows it to be administered.
Erik Baptist, attorney for the challengers, alleged that those studies all featured patients who received ultrasounds before being treated with the drug, which is not among the FDA’s requirements for prescribing abortion pills. Baptist accused the FDA of “examining oranges and declaring apples to be safe.”
Justice Department attorney Daniel Schwei defended the FDA’s approach, arguing that the relevant law gives the FDA discretion to determine what studies are adequate for approving a drug’s safety. He also said the challengers’ claims were factually flawed because the FDA also was looking at studies where the patients did not receive an ultrasound.
Impact of the reversal of Roe v. Wade: The medication abortion lawsuit targets actions the FDA took around medication abortion pills before last summer’s Supreme Court reversal of Roe v. Wade’s abortion rights protections.
While that decision, known as Dobbs v. Jackson Women’s Health Organization, didn’t play a major role in Wednesday’s arguments, the judge referenced it and suggested it could have an impact on his thinking about the case.
He asked Erin Hawley, an attorney for the challengers, whether Dobbs was an “intervening event” that has “changed the landscape” around the relationship between state and federal government concerning abortion policy. Hawley agreed, calling it a “sea change.”
What could happen next: Kacsmaryk wrapped up the hearing without any explicit timeline for when he’ll rule, telling the parties he would issue an order and opinion “as soon as possible.”
An appeal would first go to a panel of three judges of the 5th US Circuit Court of Appeals, arguably the most conservative appeals court in the country. The panel’s decision could then be appealed either to the full 5th Circuit or the US Supreme Court.
Kacsmaryk seemed to be grappling with the practical impact of a ruling in favor of the plaintiffs. He asked plaintiffs’ attorneys, the DOJ lawyers and the attorneys for the drug company Danco whether it would be possible for him to block some but not all of the FDA actions the challengers were targeting.
