
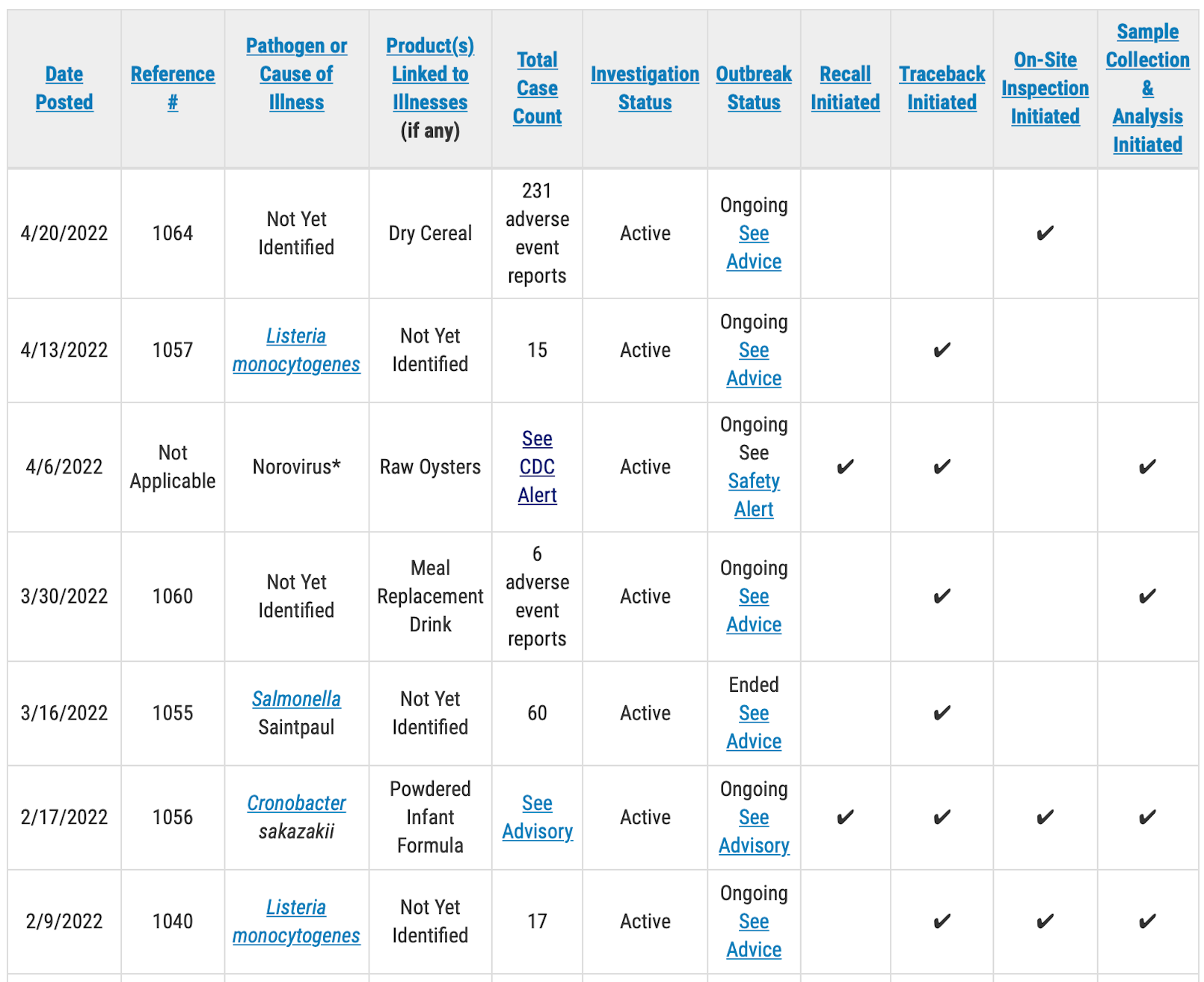
The FDA is investigating greater than 200 “hostile occasions” associated with an unnamed dry cereal. In fresh days reviews from around the nation relating to sicknesses connected to Fortunate Charms cereal were filed with executive companies and the iwaspoisoned.com website online.
The reviews come with vomiting, diarrhea and different gastrointestinal signs. The Meals and Drug Management has one by one reported in fresh days this is is investigating court cases about Fortunate Charms however has now not launched some other information about the investigation. Common Generators, the maker of Fortunate Charms, has reported that it’s not acutely aware of any showed sicknesses related to the cereal.
As of April 20, the FDA has initiated an on-site inspection on the subject of the court cases in regards to the cereal.
The company has now not reported any details about the individuals who made the court cases of inauspicious occasions associated with the cereal and has now not reported the place they reside.
In a remark launched in regards to the outbreak of “hostile occasions” connected to the “dry cereal,” the FDA states: “For hostile match document investigations, FDA will point out a product class and now not publicly title a selected product till there’s enough proof to implicate that product as a explanation for sicknesses or hostile occasions.
“For the brand new hostile occasions investigation — reference #1064 — FDA is following up on a chain of unconfirmed hostile match reviews (231 court cases) that can be related to dry cereal. Even though FDA has now not decided that this cereal is connected to those hostile match reviews, FDA is carrying out an investigation to decide the prospective causality of those court cases. The entire collection of hostile occasions reported comprises the collection of hostile occasions which have been self-reported through shoppers to FDA client criticism coordinators and the CFSAN Hostile Match Reporting Gadget (CAERS), which might come with replica reviews.”
For details about the right way to record a non-emergency meals drawback with the FDA, please click on right here.
In any other investigation into other reviews of “hostile occasions,” the FDA has revised the collection of court cases to 6, down from the 38 reported per week in the past. The company says the full collection of court cases has been diminished to just mirror the occasions reported through shoppers to the FDA’s criticism coordinators. The outbreak replace didn’t point out the place the opposite 32 court cases were filed.
The company has known the implicated product as a “Meal Substitute Drink,” however has now not reported a logo or a distribution community. The FDA has begun traceback efforts at the product in query and has begun assortment and trying out of product samples.
Different ongoing outbreaks
Federal officers are investigating a pandemic of Listeria infections and are trying out product samples on the subject of any other outbreak brought about through Listeria monocytogenes.
The brand new Listeria outbreak has sickened a minimum of 15 other people, however the Meals and Drug Management has now not but known a meals supply for the pathogen. Consistent with its same old procedure, the FDA has now not launched any details about the sufferers, equivalent to age, and has now not reported the place they reside.
As of April 20 the FDA had begun traceback however had now not undertaken any on-site inspections or pattern trying out on the subject of the outbreak.
In any other outbreak, additionally brought about through Listeria, the FDA is reporting {that a} meals supply has now not been known, nevertheless it has begun on-site inspection of an unnamed corporate. The company has begun pattern assortment and trying out, nevertheless it has now not reported what’s being examined. The affected person depend within the outbreak stays at 17.
The company additionally has ongoing investigations into a pandemic of norovirus infections traced to uncooked oysters from British Columbia, Canada, and a pandemic of Salmonella Saintpaul infections from an unknown supply. The norovirus outbreak traced to the oysters has sickened greater than 100 other people in the US and greater than 300 in Canada.
The FDA may be proceeding to research a pandemic of cronobacter infections connected to toddler formulation made through Abbott Diet that has sickened 4 young children with two deaths underneath investigation. All manufacturing on the implicated manufacturing facility in Sturgis, MI, has been stopped.
The desk beneath displays details about outbreak investigations being controlled through FDA’s CORE Reaction Groups. The investigations are in various levels. Some outbreaks have restricted knowledge with energetic investigations ongoing, others could also be close to crowning glory. The desk beneath has been abbreviated to turn handiest energetic investigations.
A public well being advisory will probably be issued for investigations that experience ended in particular, actionable steps for shoppers to take to offer protection to themselves, in line with the FDA. Please direct your consideration to these pages for the freshest knowledge at the investigation and for client coverage knowledge.
Outbreak and hostile match investigations that don’t lead to particular, actionable steps for shoppers might or won’t conclusively establish a supply or expose any contributing components. Hostile match investigations depend on self-reported knowledge. Even though those reviews might title a specific product, FDA will handiest point out a product class within the desk and won’t publicly title a selected product till there’s enough proof to implicate that product as a explanation for sicknesses or hostile occasions. If a motive and/or contributing components are known that might tell long run prevention, FDA commits to offering a abstract of the ones findings.
To view the FDA web page with hyperlinks to precise knowledge on particular person outbreaks, please click on right here.
(To enroll in a unfastened subscription to Meals Protection Information, click on right here)
